Reprogramming of pro-tumor macrophages by hydroxychloroquine in an abdominally metastasized diffuse midline glioma
Abstract
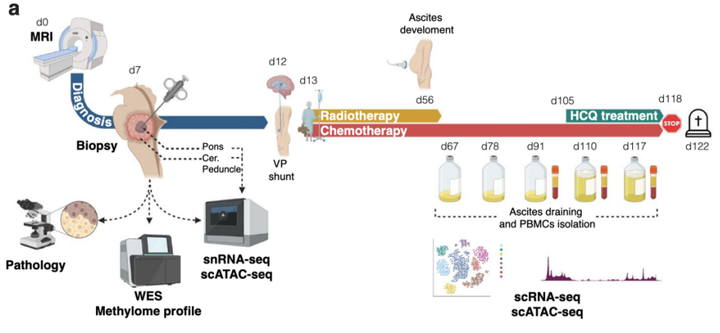
Macrophage (Mφ) repolarization from a pro-tumor, immunosuppressive phenotype towards an anti-tumor, pro-inflammatory state represents a promising therapeutic strategy in patients with cancer1. Successful reprogramming of Mφ in a clinical setting has not been documented. Here, we traced the evolution at single-cell resolution of a diffuse midline glioma (DMG) with H3K27M mutation which metastasized in the abdomen after placing a ventriculoperitoneal (VP) shunt and exploited this information for therapeutic decision-making. The primary tumor showed a complex cellular and genomic landscape characterized by heterogeneous cancer cells and a tumor-supportive immune microenvironment. Upon metastasis, malignant cells populated the peritoneum and triggered a massive anti-inflammatory immune cell infiltration and expansion of myeloid-derived suppressor cells (MDSCs) in peripheral blood. Hydroxychloroquine adjuvant treatment started to overcome the immunosuppressive milieu, which resulted in a decrease in peritoneal cancer cells and pro-tumor innate immune cells. Importantly, an emergence of anti-tumor, pro-inflammatory macrophages and cytotoxic T-cells was observed, accompanied by the activation of monocytes in the blood. Our study advocates the employment of single-cell technologies to better understand and inspire therapeutic regimens in patients with cancer.